Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:3 Percentile:29.84(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
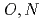 -hetero donor ligand BIZA
-hetero donor ligand BIZAKobayashi, Toru; Akutsu, Kazuhiro*; Nakase, Masahiko*; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation Science and Technology, 54(13), p.2077 - 2083, 2019/02
Times Cited Count:5 Percentile:22.52(Chemistry, Multidisciplinary)Development of separation reagent, which can efficiently separate lanthanides, is of increasing importance because it concerns establishment of purification and recycle techniques rare-earth elements. In our research group, several hetero donor type ligands, which have both oxygen and heterocyclic nitrogen atoms as donor atoms, were developed and revealed that this type of ligands show the selective complexation with specific lanthanide. In this paper, we will discuss the lanthaides complexation properties of the hetero donor type ligand containing benzimidazole group as nitrogen donor ( -methyl-
-methyl- -phenyl-2-(1
-phenyl-2-(1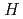 -benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
-benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
Separation Science and Technology, 52(7), p.1186 - 1192, 2017/03
Times Cited Count:2 Percentile:9.15(Chemistry, Multidisciplinary)The new extractant, biuret(C8), is synthesized and tested for the solvent extraction of hard acid metals like actinides and soft acid metals. This compound has the similar central frame to malonamide but with 2-NH introduced into the central frame, then both amidic oxygen and nitrogen atoms may bond with metals. From the present work, not only hard acid metals, but also soft acid metals can be extracted by biuret(C8) from nitric or perchloric acids to n-dodecane. The extractability for biuret(C8) is compared with other representative extractants, malonamide, TODGA and MIDOA. It is clear that the distribution ratio(D) of U and Pu by biuret(C8) is similar to those for malonamide, but with lower values than those for TODGA and MIDOA, and D for soft acid metals and oxonium anions show higher values than those for TODGA and malonamide and lower than those for MIDOA.
Suzuki, Tomoya; Morita, Keisuke; Sasaki, Yuji; Matsumura, Tatsuro
Separation Science and Technology, 51(17), p.2815 - 2822, 2016/09
Times Cited Count:8 Percentile:23.23(Chemistry, Multidisciplinary)To understand the adsorption properties of styrene-divinylbenzene copolymer functionalized with 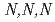 -trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and
-trimethylglycine, AMP03, the adsorption behaviors for platinoid ions (Ru(III), Rh(III), and Pd(II)) were examined. Furthermore, we performed adsorption experiments using sample solutions with adding triethylamine, thiourea, and 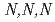 -trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
-trimethylglycine. Based on the adsorption data obtained in this study, we performed chromatographic experiments. The results indicated that all platinoid ions in the feed solution completely adsorbed on AMP03, and almost 80% of the adsorbed platinoid ions were recovered. These results show that AMP03 has the potential to recover Ru(III), Rh(III), and Pd(II) from high-level liquid waste.
 ,
, -di(2-ethylhexyl)butanamide-nitric acid systems using turbidity measurements
-di(2-ethylhexyl)butanamide-nitric acid systems using turbidity measurementsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Matsumura, Tatsuro
Separation Science and Technology, 51(6), p.961 - 967, 2016/02
Times Cited Count:1 Percentile:4.78(Chemistry, Multidisciplinary)Quantitative evaluation of the two-phase separation between  ,
, -di(2-ethylhexyl)butanamide (DEHBA) and tri-
-di(2-ethylhexyl)butanamide (DEHBA) and tri- -butyl phosphate (TBP) diluted with
-butyl phosphate (TBP) diluted with  -dodecane and uranyl nitrate solution in nitric acid medium was achieved using turbidity measurements. The turbidities of DEHBA were relatively high, particularly at high DEHBA concentrations, while that of TBP rapidly decreased irrespective of nitric acid concentration. A high concentration of DEHBA, nitric acid, and uranium increased the turbidities in the organic phase, which could be ascribed to the increase in viscosity. Distribution ratios of uranium were also measured, and it was indicated that turbidity did not have a critical effect on the distribution ratio when the turbidity was below a certain value.
-dodecane and uranyl nitrate solution in nitric acid medium was achieved using turbidity measurements. The turbidities of DEHBA were relatively high, particularly at high DEHBA concentrations, while that of TBP rapidly decreased irrespective of nitric acid concentration. A high concentration of DEHBA, nitric acid, and uranium increased the turbidities in the organic phase, which could be ascribed to the increase in viscosity. Distribution ratios of uranium were also measured, and it was indicated that turbidity did not have a critical effect on the distribution ratio when the turbidity was below a certain value.
Nakase, Masahiko*; Takeshita, Kenji*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation Science and Technology, 50, p.2832 - 2835, 2015/05
Times Cited Count:1 Percentile:5.02(Chemistry, Multidisciplinary)Separation of trivalent lanthanides (Ln) and trivalent actinides (An) is important task in reprocessing high-level liquid waste. In this study, the relationship between structures of complexes of 2,2'-bipyridyl (Bpy) and Ln and coordination strengths were investigated by X-ray diffraction and UV titration. With the decrease in ionic radius from Nd to Er, the N(Bpy)-Ln distance and N(Bpy)-Ln-N(Bpy) angle decreased, the dihedral angle of the Bpy pyridines increased, and the stability constant increased. This information about structure and coordination strength is important for designing better ligands for separating Ln and An ions.
Tanaka, Ryota*; Ishihara, Ryo*; Miyoshi, Kazuyoshi*; Umeno, Daisuke*; Saito, Kyoichi*; Asai, Shiho; Yamada, Shinsuke*; Hirota, Hideyuki*
Separation Science and Technology, 49(1), p.154 - 159, 2014/01
Times Cited Count:4 Percentile:16.82(Chemistry, Multidisciplinary)Koyama, Shinichi; Suto, Mitsuo; Obayashi, Hiroshi; Takeshita, Kenji*; Ogata, Takeshi*; Oaki, Hiroshi*; Inaba, Yusuke*
Separation Science and Technology, 47(14-15), p.2024 - 2028, 2012/11
Times Cited Count:3 Percentile:15.89(Chemistry, Multidisciplinary)A series of separation experiments was performed to study the recovery process for minor actinides (MAs) from the actual spent fuel by using an extraction chromatographic technique. TPPEN gel was used as a new extraction chromatographic agent. Mixed oxide fuel was used as a reference spent fuel to demonstrate the recovery of the MAs. The MOX fuel, including 29.9 wt% plutonium (Pu), was irradiated up to 119 GWd/MTM, and the fuel was then prepared for the extraction experiment. A Mixed solution of MAs and lanthanides (Lns) was prepared. The TPPEN gel was immersed in a 0.01 M NaNO solution, and the pH was adjusted to 4.0. Next, an extraction column was prepared using the TPPEN gel, and the mixed solution of MAs and Lns was passed through the column. The Lns were detected in the eluent after washing with 0.01 M NaNO
solution, and the pH was adjusted to 4.0. Next, an extraction column was prepared using the TPPEN gel, and the mixed solution of MAs and Lns was passed through the column. The Lns were detected in the eluent after washing with 0.01 M NaNO (pH 4.0). For detecting the MAs, the pH of the eluent was changed to 2.0.
(pH 4.0). For detecting the MAs, the pH of the eluent was changed to 2.0.
Kobayashi, Toru; Yaita, Tsuyoshi; Suzuki, Shinichi; Shiwaku, Hideaki; Okamoto, Yoshihiro; Akutsu, Kazuhiro*; Nakano, Yoshiharu*; Fujii, Yuki*
Separation Science and Technology, 45(16), p.2431 - 2436, 2010/11
Times Cited Count:35 Percentile:71.17(Chemistry, Multidisciplinary)Fugate, G.*; Takeshita, Kenji*; Matsumura, Tatsuro
Separation Science and Technology, 43(9&10), p.2619 - 2629, 2008/07
The compound N,N,N',N'-tetrakis(methylpyridyl)-1,2-ethylenediamine (TPEN) has been shown previously to exhibit a high separation factor for the separation of americium(III) and europium(III) but the application of this material to the nuclear fuel cycle is limited because of pH range of the separation (pH3 to 6) and the high solubility of the compound in acidic aqueous media. A variety of compounds have been synthesized during the course of this study in an attempt to retain the separation factor found in the parent compound while decreasing the working pH and aqueous solubility of the extractant. A series of compounds was synthesized in which the ethylene linker was replaced by several aromatic compounds. Another series of analogs was synthesized replacing two or more of the methylpyridyl groups with several different functional moieties. The separation properties of these new compounds were characterized and will be presented.
Kavakli, P. A.*; Seko, Noriaki; Tamada, Masao; G ven, O.*
ven, O.*
Separation Science and Technology, 39(7), p.1631 - 1643, 2005/00
Times Cited Count:58 Percentile:84.37(Chemistry, Multidisciplinary)A new type of fibrous adsorbent with excess amidoxime groups was synthesized by radiation-induced graft polymerization. Glycidyl methacrylate (GMA) was first radiation-grafted on polyethylene-coated polypropylene nonwoven fabrics and chemically modified with 3,30-iminodipropionitrile [NH (-CH -CH
-CH -CN)
-CN) ] (IDPN), which was further reacted with hydroxylamine to obtain graft chains containing two amidoxime groups per graft repeating units. The adsorption properties of this new adsorbent for uranium (U), vanadium (V), lead (Pb), copper (Cu), and cobalt (Co) ions at low concentrations (3.3-1000 ppb).
] (IDPN), which was further reacted with hydroxylamine to obtain graft chains containing two amidoxime groups per graft repeating units. The adsorption properties of this new adsorbent for uranium (U), vanadium (V), lead (Pb), copper (Cu), and cobalt (Co) ions at low concentrations (3.3-1000 ppb).
Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Separation Science and Technology, 40(16), p.3349 - 3364, 2005/00
Times Cited Count:3 Percentile:20.23(Chemistry, Multidisciplinary)Poly-glycidyl methacrylate (GMA) was appended onto a porous membrane of a hollow-fiber form. Octadecylamine was added to the epoxy group of the polymer brush at a maximum molar conversion of the epoxy group into the octadecylamino group of 59%. Bis(2,4,4,-trimethylpentyl)phosphinic acid (Cyanex 272), was impregnated on the octadecylamino group by immersion of the octadecylamine-added porous membrane. The phosphinic acid moiety of Cyanex 272 was attracted by the amino part of the octadecylamino group of the polymer brush to swell the entire volume of the porous membrane. The impregnated Cyanex 272 was repositioned on the charged polymer brush in response to the properties of surrounding liquids. The hydrophobic interaction enables the hydrophobic moiety of Cyanex 272 to associate with the octadecyl part of the octadecylamino group of the polymer brush to capture zinc ions. The binding efficiency of the Cyanex 272-impregnated polymer brush for zinc ion was as high as 93%.
Seko, Noriaki; Katakai, Akio; Tamada, Masao; Sugo, Takanobu*; Yoshii, Fumio
Separation Science and Technology, 39(16), p.3753 - 3767, 2004/00
Times Cited Count:83 Percentile:89.98(Chemistry, Multidisciplinary)Fibrous amidoxime adsorbents were prepared by radiation-induced co-grafting of acrylonitrile (AN) and methacrylic acid (MAA) and subsequent amidoximation. Adsorption of uranium in seawater was evaluated by pumping seawater into the adsorbent column. The best monomer ratio of AN and MAA was 7:3 for continual usage of uranium adsorption. Though hydrochloric acid is an effective eluting agent for the metals adsorbed on the adsorbent, amidoxime groups were simultaneously damaged after five cycles of adsorption-desorption. This deterioration was reduced by an alkaline treatment of the adsorbents after each elution. Furthermore, various organic acids were examined as elution agents. It was found that the 80% of adsorption activity was still maintained after five cycles of adsorption-desorption when tartaric acid was used for eluting agent.
Mineo, Hideaki; Goto, Minoru; Iizuka, Masaru*; Fujisaki, Susumu; Hagiya, Hiromichi*; Uchiyama, Gunzo
Separation Science and Technology, 38(9), p.1981 - 2001, 2003/05
Times Cited Count:22 Percentile:63.74(Chemistry, Multidisciplinary)no abstracts in English